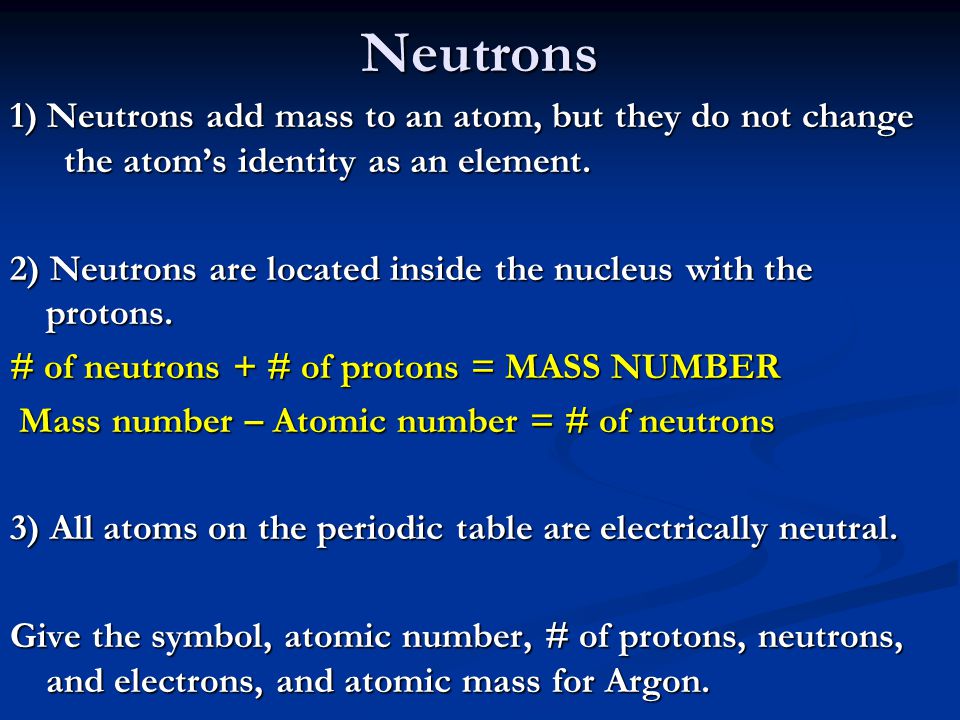
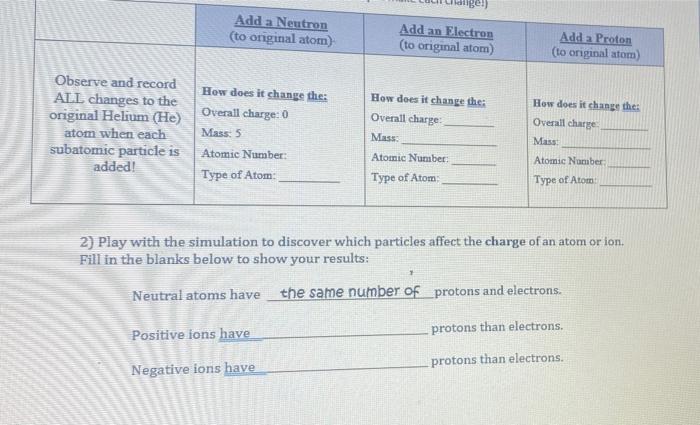
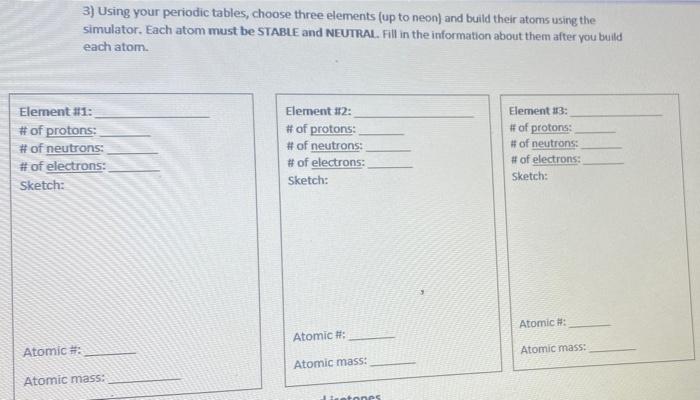
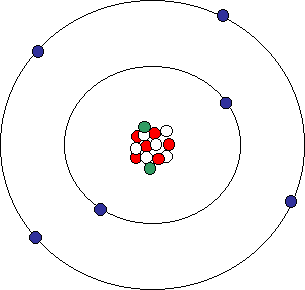
Biology, The Chemistry of Life, The Chemical Foundation of Life, Atoms, Isotopes, Ions, and Molecules: The Building Blocks | OERTX

Adding neutrons to synthetic atoms drastically alters shape of their nuclei, affects their stability
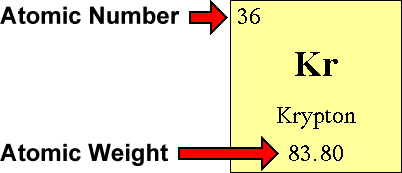
Questions and Answers - How do I find the number of protons, electrons and neutrons that are in an atom of an element?

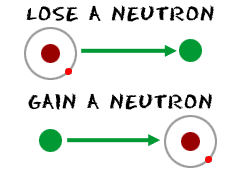
True or false? Adding one neutron to the nucleus of an atom increases its atomic number by one unit but does not change its atomic mass. | Study.com

SOLVED:Consider an atom of 10 $\mathrm{B}$ . (a) How many protons, neutrons, and electrons does this atom contain? (b) What is the symbol of the atom obtained by adding one proton to $^{

SOLVED:Consider an atom of 10 $\mathrm{B}$ . (a) How many protons, neutrons, and electrons does this atom contain? (b) What is the symbol of the atom obtained by adding one proton to $^{

True or false? Adding one neutron to the nucleus of an atom converts it to an isotope of the same element. | Study.com